What is absorption
Adsorption of activated carbon is the accumulation of adsorbate molecules on the surface of the adsorbent, and the amount of adsorbable material depends on the specific surface area of the adsorbent.
The pores of activated carbon represent a complex mesh structure and have capillaries forming a huge internal surface area.
① Activated carbon pore structure and pore distribution

② Classification by pore type
| Type | Size | Content |
|---|---|---|
| macropore | 1000Å or bigger | big effective radius, Take in adsorbates from outside |
| mesopore | 20~1000Å | capillary condensation occurs |
| micropore | 20Å or lower | the smallest radius, occupying 95% of specific surface area |
③ Activated carbon material property
| Raw material | Specific surface area(㎡/g) | Pore capacity(㎖/g) | Average pore diameter)(㎚) |
|---|---|---|---|
| Charcoal group(chemical revival) | 1,120 | 1.03 | 3.0 |
| Coal group | 1,050 | 0.55 | 1.4 |
| Coconut group | 1,050 | 0.45 | 0.9 |
| Coal group(chemical revival) | 1,020 | 1.02 | 3.0 |
④ Absorption mechanism
Adsorption occurs at the interface where two phases are in contact, and the density of each phase is different from the inside. For example, in a solution, a phenomenon in which the concentration of a solute in an interface portion in contact with a solid or a surface portion in contact with air is different from the concentration of a solute in a liquid is called adsorption. In addition, a substance that provides a surface to allow molecules to adhere is called an adsorbent, and a molecule attached to the surface is called an adsorbate. The adsorbent is porous and has a very large internal surface and a solid adsorbent.
Adsorption is a three-step reaction to collect dissolved substances in solution on the surface of the adsorbent.
| Step 1 | Adsorbates move to the outer surface of the adsorbent (activated carbon) (slow) |
|---|---|
| Step 2 | Adsorbates diffuse through the large and medium pores of activated carbon (slow) |
| Step 3 | Diffused adsorbates are bound to the inner surface of the micropore or fill the micropore (fast) |
Since Step 3 of the above steps occurs quickly, adsorption is limited mainly in steps 1 and 2.
Therefore, it can be seen that the adsorption rate is determined by the rate at which the molecules of the adsorbate move, ie diffuse, in solution.
Eventually, steps 1 and 2 become the limiting step. In the ash adsorption, since the forcing force occurs due to agitation, the adsorbed material has a large movement speed due to turbulence and diffusion, and the limiting sep of the initial process varies depending on the type of the adsorbent and the degree of diffusion due to the movement speed of the molecule. The model of adsorption is shown in the figure below.
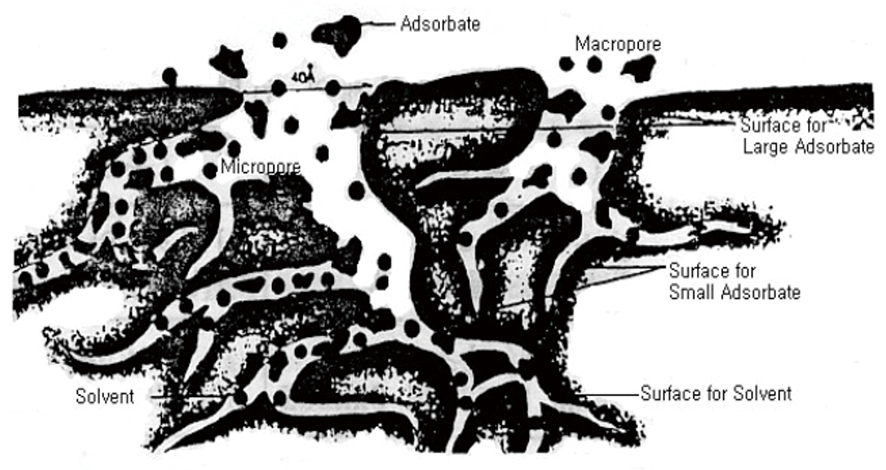
⑤ Types of adsorption
| Classification | Physical adsorption | Chemical adsorption |
|---|---|---|
| Binding | Weak binding by Vander Walls pulling | Strong ion or covalent binding by rearrangement of free electrons |
| Temperature | The hotter, the less the adsorption | Adsorption increases as the temperature increases and at some point starts to decrease |
| Adsorbate | Adsorb all gases below the critical temperature (multiple adsorption) | Selectively adsorb chemically responsive adsorbates (single adsorption) |
| Adsorption heat | Small. Equal to condensation heat (10Kcal/Mole or lower) | Big. Equal to reaction heat (10~30Kcal/Mole) |
| Adsorptio speed | Fast(no activation) | Slow(activation energy needed) |
| Reversibility | Always reversible (easy desorption even at around 150℃) | Reversible or irreversible (easy desorption at 800℃ or higher) |